Scientific project description
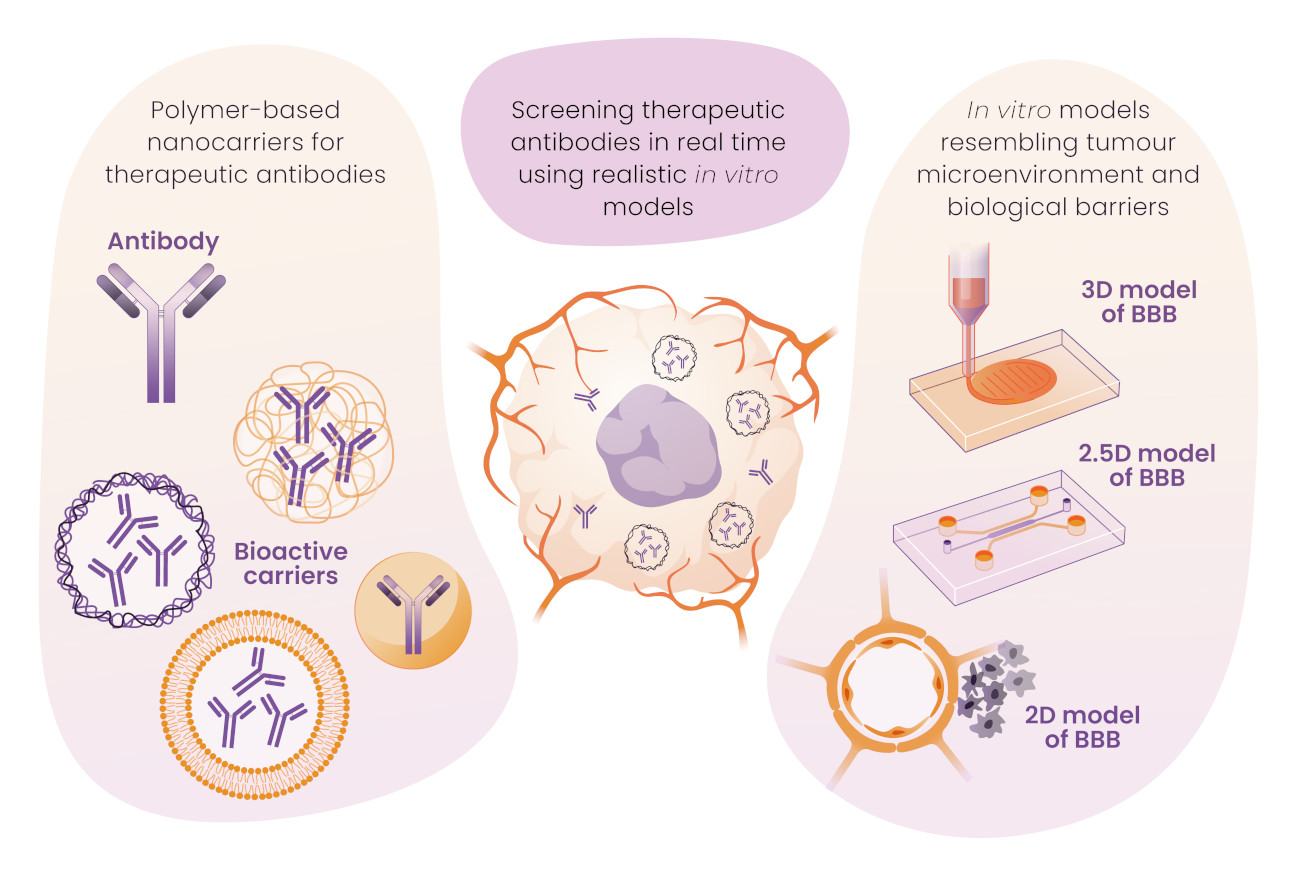
The development of therapies against cancer demands huge scientific and human resources and, even so, most of the projects fail due to the extreme difficulty of reaching, treating, and testing the effect of the therapeutic drugs on cancer cells due to the complexity of the tumor microenvironment. This situation becomes even more challenging when the malignant cells are located in the brain. For this reason, albeit glioblastoma (GBM) constitutes just the 2% of the brain tumors, it leads to the 7% of total cancer deaths. One of the main justifications for such aggressive effect of GBM is the presence of a biological barrier, known as Blood Brain Barrier (BBB), that hinders the extravasation of most therapeutics to their target tissue. Hence, GBM considered, researchers working in drug delivery systems need to reformulate and adapt their therapeutic carriers to enable the BBB crossing. In addition, all the GBM models that are built to test the efficiency of the therapeutic agents should also include a BBB-like protective layer, which increases the complexity of the system notoriously. Therefore, the presence of the BBB compels the researchers to apply complex scientific resources to develop a therapeutic drug against GBM.
TheraTools project aims to provide the scientific community with novel means to face the main issues that are intrinsic to the brain diseases. Moreover, TheraTools, following the principles of the MSCA Green Charter, is committed to prompt the implementation of realistic tumor models that will eventually replace in-vivo models, minimising thereby the environmental footprint caused by our investigations. Thus, this research project covers most of the stages of the development and trials of therapeutic agents, from its chemical formulation to its assessment in advanced tridimensional models and validation in in-vivo models.
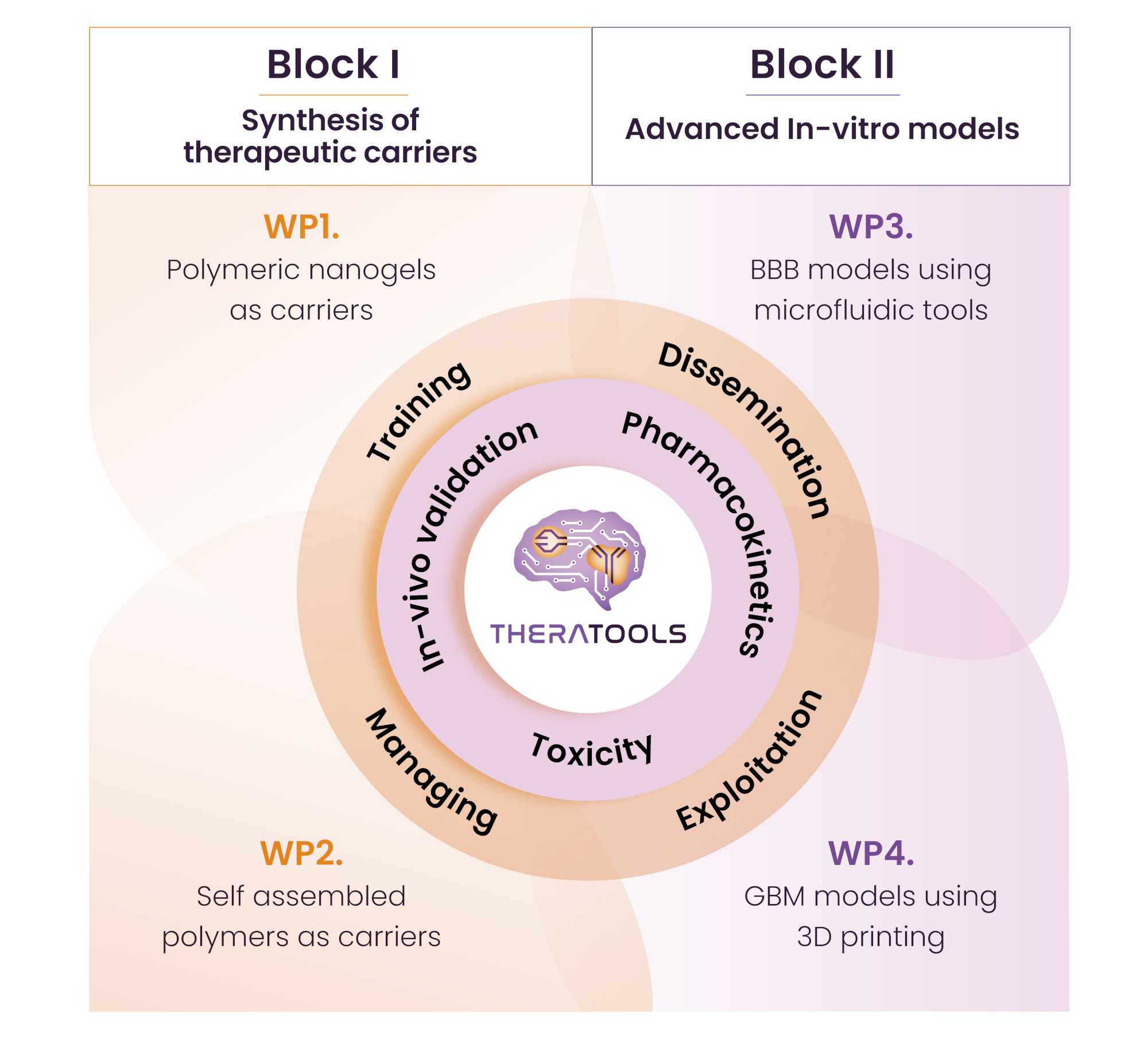
Figure: Scientific work packages of TheraTools are transversally interconnected and encompassed in 2 research Blocks.
Thus, TheraTools encompasses the achievement of three ambitious goals:
- The development of novel carriers able to cross the BBB and release the therapeutic agent in presence of the biological target, i.e. GMB). Guided by reported encouraging results, we have chosen a monoclonal antibody, i.e. cetuximab (CemAb), as the therapeutic model drug that will be used in this project. Our approach considers the embedment of CemAb within a synthetic/natural carrier. This platform will include the chemical signals to cross the BBB and the specific responsiveness to release the biomacromolecule only in the environment of the GBM.
- The development of an in vitro BBB model applying microfluidics. A huge effort will be given to the design and fabrication of valid BBB models so they can recapitulate those events happening in a real in vivo situation, using current microfluidic tools. These testing models will be the first validation assays of the therapeutics developed in the first objective.
- The development of 3D glioblastoma models. In this objective, realistic 3D GMB that include the BBB will be fabricated. These testing models will be the second validation assays of the therapeutics developed in the first objective.
Finally, the project accounts the validation of the most promising agents in in vivo GMB models.
